The German Supply Chain Due Diligence Act (SCDDA) and its Impact on the Due Diligence Requirements in the Pharma Supply Chain
In force since January 1, 2023 and initially applicable to all companies with a registered office or subsidiary in Germany having more than 3,000 employees, the German Supply Chain Due Diligence Act (SCDDA) has broadened its scope from January 1, 2024 – when the threshold of employees was lowered to 1,000. Nevertheless, many small and medium-sized enterprises could also be de facto affected by the SCDDA because they are (direct) suppliers and are therefore contractually required by their customers to comply with due diligence obligations – in order to prevent or at least minimize human rights and environmental rights infringements in their supply chains.
As a result, this new local regulation expands well beyond the German market with a major impact on all the larger companies who predominantly act globally, such as many of the companies in the corporate life science and health care industry.
Effects on the Pharma Supply Chain:
- Compliance Challenges
A transparent supply chain, independent from the sector, is vital in improving environmental and social footprints and ensuring compliance with the German Supply Chain Due Diligence Act.
In the pharma industry, numerous international companies have been (proactively) enhancing their governance and compliance management capacities and investing in technologies that easily and automatically gather data on suppliers’ environmental and social practices – safeguarding that appropriate due diligence measures are taken and documented throughout the entire supply and value chain.
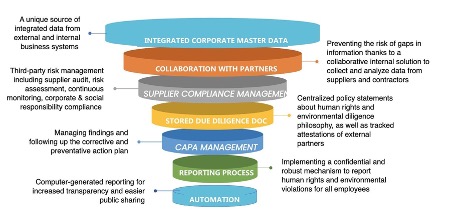
Figure: Essential requirements for achieving compliance with SCDDA
Source: The German Supply Chain Due Diligence Act and its Impact on the Pharma Supply Chain – Kvalito
- Integrity Management
Integrity management is an evolving area that assists corporations on how to implement:
- the highest ethical standards of product quality
- open communication and transparency
Currently, to complement the existing regulations and to offer a fully ethical view of the organization from within, pharmaceutical companies implement different models, e.g., the Balanced Scorecard, Lean Six Sigma, and the Triple Bottom Line (“people, planet, prosperity”).
The pharma industry’s very purpose is to deliver medicines to improve patients’ lives (“People“). The management of manufacturing facilities is highly committed to environmental compliance and constantly striving to cut waste and optimize productivity (“Planet“). However, fulfilling their purpose as a business to produce drugs also generates significant profit (“Prosperity“).
- Reputational Impact
Companies are more and more scrutinized for unethical behaviour, including corruption, labour issues, poor transparency and accountability, inadequate corporate integrity systems and poor working conditions in their own operations, in those of their subsidiaries, as well as for the actions of subcontractors acting on their behalf.
As consumers, patients and shareholders are progressively inclined to recognize the value of interrelated corporate responsibility and sustainability in international business, numerous pharma businesses have embraced the social and environmental agenda in recent years.
- Business Considerations
Important elements for the pharmaceutical companies to consider:
- The financial market and business customers/patients are also increasingly demanding evidence of compliance with the relevant standards.
- Many international companies have been partnering with EcoVadis, the world’s most trusted provider of business sustainability ratings.
- Transparency requirements specific to large enterprises are also relevant for medium-sized companies.
- Companies that fulfil these requirements particularly well improve their attractiveness as suppliers.
- Managing relevant sustainability topics along the supply chain is essential for future competitiveness.
Businesses that do not accomplish their due diligence and reporting obligations regarding human rights and environmental standards in their supply chains face fines of up to €8 million, depending on the type and severity of the violation. These companies also face reputational damage and could be excluded from the award of public contracts for up to three years.
For further information, please read the following:
The German Supply Chain Due Diligence Act and its Impact on the Pharma Supply Chain – Kvalito
and
Deloitte-SCDDA-implementation-companies-EN.pdf